PLEASE MATCH YOUR ASSIGNMENT QUESTIONS ACCORDING TO YOUR SESSION
IGNOU CHE-10 (January 2025 – December 2025) Assignment Questions
1. What is Rydberg constant? Calculate its value. Why is its experimental value different from the calculated value?
2. Derive the symbol for the ground state of hydrogen atom.
3. Write the symmetry elements and the point groups of the following:
(i) Water (ii) Ammonia (iii) Benzene
5. The transition from J = 0 to J’ = 1 for HCl takes place at v = 21.18 cm-1. Calculate the bond length for 1H35Cl.
6. What is anharmonicity? Draw a diagram for the variation of potential energy with intermolecular distance for an anharmonic and harmonic oscillator
7. Draw and explain the normal modes of vibration of water molecule.
8. Which functional groups are expected to absorb in the 2000 – 1500 cm-1 region of IR spectrum? Explain.
9. Discuss the pure rotational spectrum of CO2 using the suitable diagram
10. Give the selection rules and the schematic diagram of the vibration-rotation Raman spectrum of a diatomic gas.
11. Derive the term symbol for the ground state of LiH.
12. How does solvent polarity affect π-π*and n-π* transitions? Explain.
13. Give reason for the following:
(a) Mercury (II) iodide is brick red in colour.
(b) The aqueous solution of NiSO4 which is pale green turns deep blue on addition of ethylenediamine
14. Answer the following:
(i) What is the role of littron mirror in IR spectrometer?
(ii) Why glass cell cannot be used for IR spectroscopy?
(iii) What are the basic requirements of any container which holds the sample?
(iv) What is the role of Water jacket in Raman Spectrometer?
(v) What is the role of Golay cell in IR spectrometer
15. Draw and explain the NMR spectrum of CH3CHO.
16. The hydrogen atoms attached to a double bond are deshielded while in case of alkynes the protons are shielded. Explain.
17. Draw and explain the important components of an ESR spectrometer.
18. Discuss the ESR spectrum of ethyl radical.
19. What is simple cleavage? Illustrate with the help of a suitable example.
20. Discuss the expected spectral data for C6H5CH2OH.
IGNOU CHE-10 (January 2024 – December 2024) Assignment Questions
1. Draw a labeled diagram for various series of spectral lines observed in the atomic spectrum of hydrogen atom.
2. Derive the spectroscopic states of carbon atom.
3. Illustrate the axis/axes of symmetry present in the following molecules:
(i) H2O (ii) NH3 (iii) BF3 (iv) benzene
4. Which elements of symmetry are present in the following molecules?
(i) PCl5 (ii) C2H2
Also give the point groups of these molecules.
5. Show transitions between various rotational levels and spectral lines arising from such transitions for a rigid diatomic molecule. What is the difference between various transitions?
6. (a) Explain the following terms:
(i) Zero point energy
(ii) Fundamental transitions
(iii) First overtone
(b) What is Morse potential? Briefly discuss.
7. Discuss the IR spectrum exhibited by water molecule. Also draw the suitable diagram.
8. Explain the following:
(i) Benzophenone absorbs at 1700 cm-1 while acetone absorbs at 1720 cm-1 in their IR spectra.
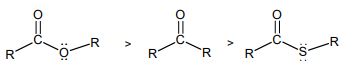
(ii) The IR absorptions frequencies of the following compounds are in the order shown below:
9. How do the stokes, anti-stokes and Rayleigh lines appear in the Raman spectrum? Explain giving the suitable diagram.
10. Explain the IR bands exhibited by CO2 and SO2 in the light of mutual exclusion principle and arrive at the structures of these molecules.
11. Define the following terms:
(i) Auxochrome
(ii) Chromophone
(iii) Hypsochromic shift
(iv) Hypochromic effect
(v) Hyperchromic effect
12. Discuss the effect of change of solvent from polar to non-polar on the π→π∗ and n→π∗ transitions.
13. Draw the crystal field splitting of d-orbitals of metal ions in complexes having different geometries.
14. Briefly explain fluorescence using a suitable diagram.
15. Draw the back diagram of a single beam IR spectrometer. How does a double beam instrument different from that of the single beam IR spectrometer?
16. Show that the energy difference between two spin states of a nucleus is given by
ΔE =| gN |BNBZ
17. Draw and explain the N,M,R spectrum of CH3CHO molecule.
18. Draw and explain the functioning of an ESR spectrometer.
19. What is simple cleavage? Illustrate it with the fragmentation of a suitable molecule.
20. Explain spectral signals expected in the different spectra of benzyl alcohol. What different units of the molecules are responsible for them?







